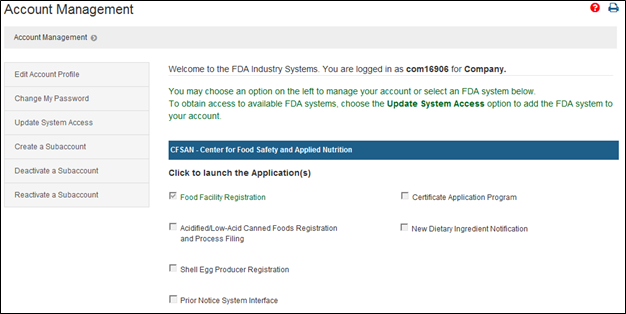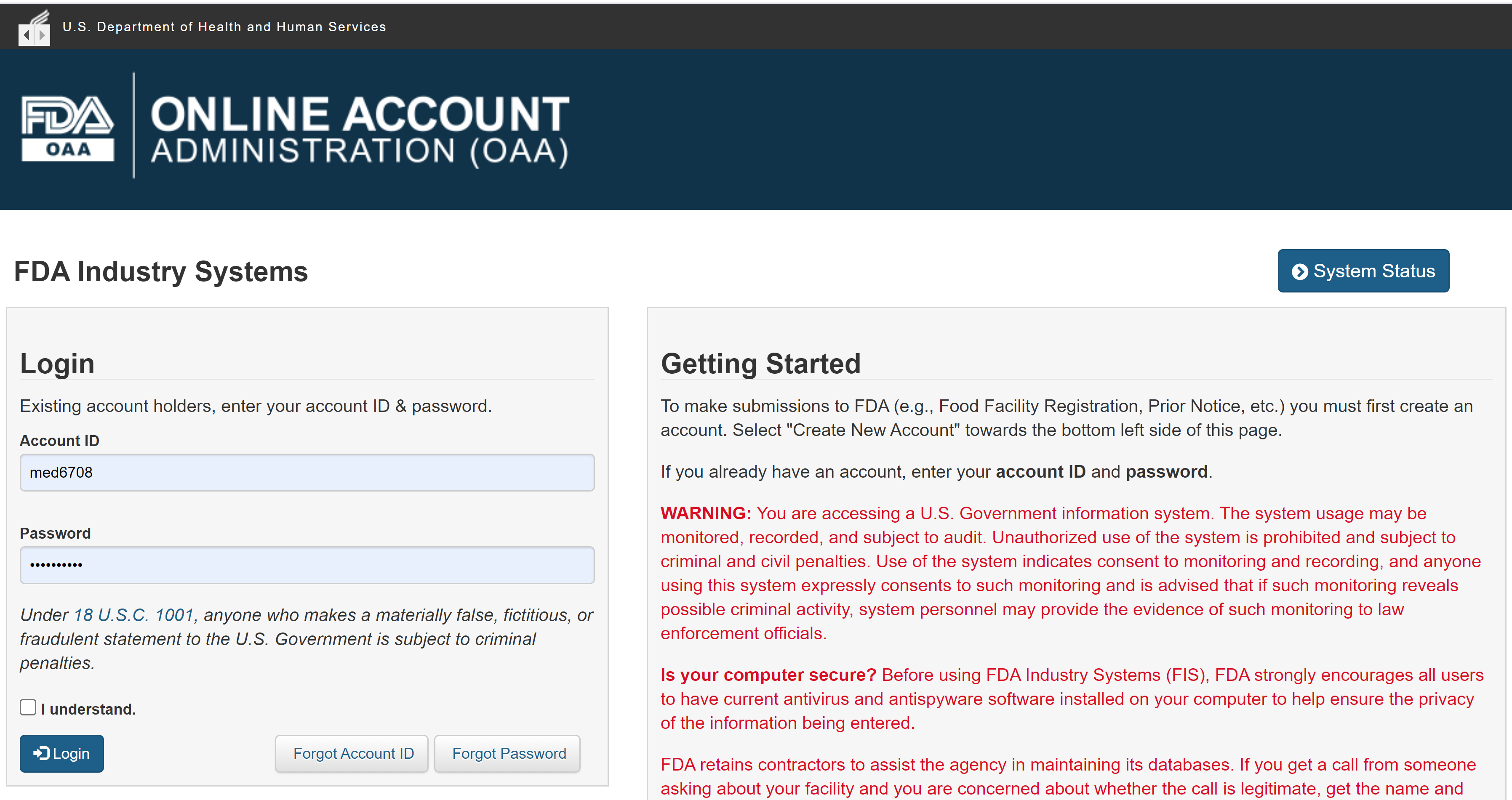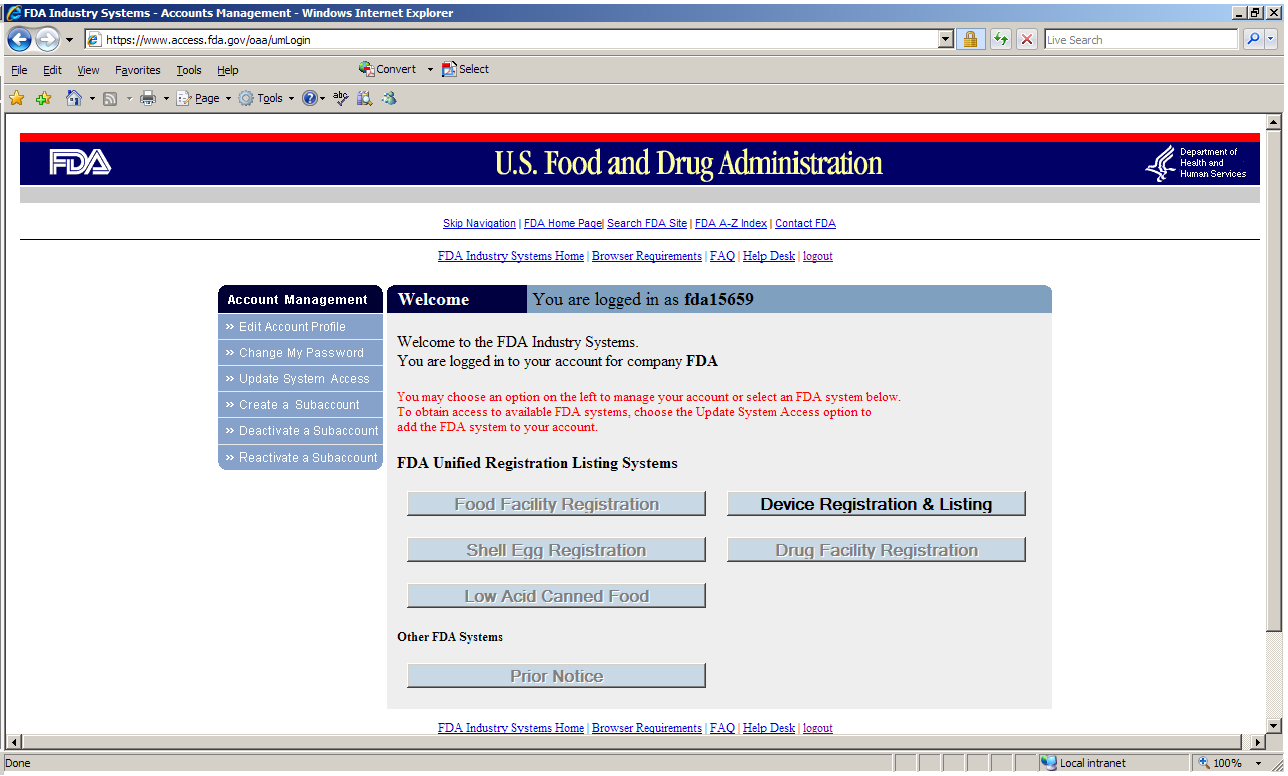
28 avril 2023 : Microbiome Post - MaaT Pharma Announces U.S. FDA Lifts Clinical Hold on Phase 3 Investigational New Drug Application for MaaT013 in Patients with Acute Graft-versus-Host Disease (English only) - MaaT Pharma

The FDA and Worldwide Quality System Requirements Guidebook for Medical Devices: 9780873893770: Medicine & Health Science Books @ Amazon.com

Veolia won the "first gold"! What made the first FDA certification in the China's plastic recycling industry so special? | Veolia China

New | Cybersecurity in Medical Devices | Quality System Considerations and Content of Premarket Submissions

FDA Drug Information on X: "FDA issued a final guidance that provides industry, investigators and others recommendations on the use of digital health technologies (DHTs) to acquire data remotely from participants in